The recommended starting oral dose of Sensipar® is 30 mg twice daily in adult patients with primary HPT with severe hypercalcemia (calcium [Ca] > 12.5 mg/dL).1
- The dose of Sensipar® should be titrated every 2 to 4 weeks through sequential doses of 30 mg twice daily, 60 mg twice daily, 90 mg twice daily, and 90 mg 3 or 4 times daily as necessary to normalize serum calcium levels
- Sensipar® must be taken orally, and should be taken with food or shortly after a meal
- The tablets should always be taken whole and not chewed, crushed, or divided.
Serum calcium should be measured within 1 week after initiation or dose adjustment of Sensipar®. Once maintenance dose levels have been established, serum calcium should be measured every 2 months.
The safety profile of Sensipar® in these patient populations is generally consistent with that seen in patients with chronic kidney disease (CKD) on dialysis.
- The most commonly reported side effects of Sensipar® in a clinical study of patients with primary HPT were nausea (59%), vomiting (35%), and paresthesia (29%)1,2
- Treatment-related hypocalcemia (< 8.4 mg/dL) occurred in 2 patients (12%)2
- Four patients (24%) withdrew from the study because of adverse events2
Sixty-seven patients with primary HPT who met criteria for parathyroidectomy on the basis of corrected total serum calcium (> 11.3 mg/dL [2.82 mmol/L] and ≤ 12.5 mg/dL [3.12 mmol/L]), but who were unable to undergo parathyroidectomy participated in a randomized, double-blind, placebo-controlled study. A total of 33 patients were randomized to Sensipar® and 34 patients randomized to placebo. The mean age of the patients was 72 years, 52% were female, 61% were Caucasian, and 5% were Blacks. The study started with a 12-week titration phase, followed by a 16-week efficacy assessment phase. Cinacalcet was initiated at a dose of 30 mg twice daily and titrated to maintain a corrected total serum calcium concentration within the normal range. During the efficacy period a significantly higher percentage of cinacalcet-treated patients compared with the placebo-treated patients achieved mean corrected total serum calcium concentration(≤ 10.3 mg/dL [2.57 mmol/L], 75.8% vs 0%, P < 0.001) and ≥ 1 mg/dL [0.25 mmol/L] decrease from baseline in mean corrected total serum calcium concentration (84.8% vs 5.9%, P < 0.001). The median dose of Sensipar® at the completion of the study was 60 mg/day.
In a randomized double-blind, placebo-controlled study of 67 patients with primary hyperparathyroidism for whom parathyroidectomy would be indicated on the basis of serum calcium levels, but who are unable to undergo surgery, the most common adverse reactions were nausea (30%), muscle spasms (18%), headache (12%), and back pain (12%).
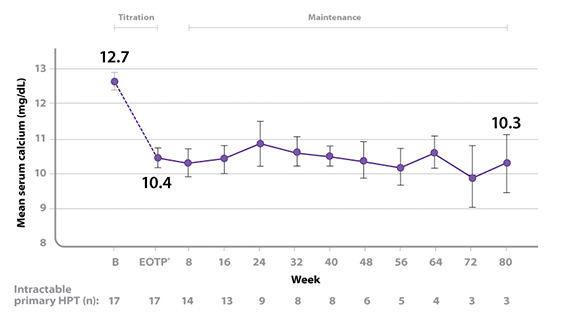
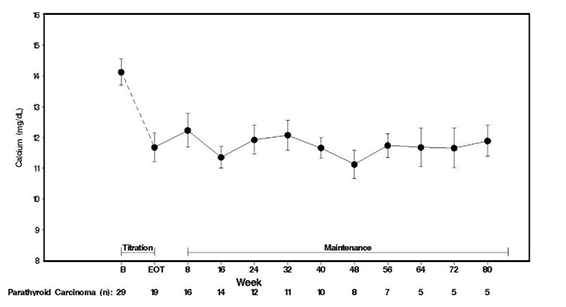
B = baseline; EOTP = end of titration phase.
Normal range as defined by Marcocci and colleagues was calcium 8.4–10.3 mg/dL.
Adapted from Marcocci et al, J Clin Endocrinol Metab, 2009.
Seventeen patients entered the study. The median exposure to cinacalcet was 270 days (range: 32 to 1105). At baseline the mean (SE) serum calcium was 12.7 (0.2) mg/dL. At the end of the titration phase the mean (SE) serum calcium was 10.4 (0.3) mg/dL, which is a mean reduction of 2.3 (0.3) mg/dL from baseline. The graph above illustrates mean serum calcium (mg/dL) over time for all patients still on study at each time point from the beginning of titration to study visit week 80. Daily dose during the study ranged from 30 mg twice a day to 90 mg 4 times a day.1
- 88% (n = 15) of patients treated with Sensipar® achieved a reduction of ≥ 1 mg/dL in serum calcium at the end of the titration period2